Peptide bonds refer to the covalent bonds that hold protein molecules together. In simple terms, they are the bonds responsible for holding amino acids together to lead to the formation of peptide chains, and these chains are then joined together to form proteins. Covalent bonds on the other hand, are formed when one atom within a molecule shares more than one electron with an atom belonging to another molecule. Covalent bonds are very strong and extremely difficult to break under normal circumstances.
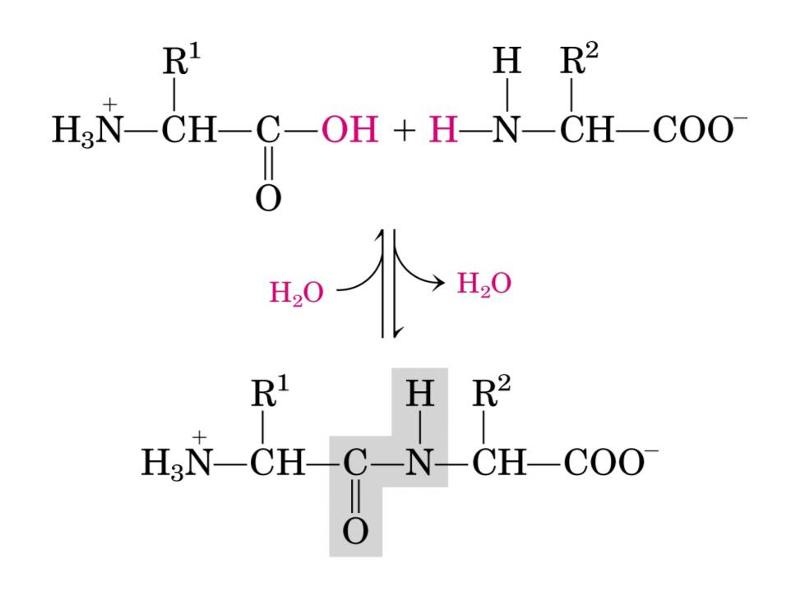
All proteins made from amino acids are joined together by the bonds in very specific ways. Most of the amino acids will have what is known as carboxyl group on one side, and on the other side, you will find an amino acid. When one carboxyl group joins next to the amino acid, a peptide bond is formed, and this is what will hold the two together.
During the formation of peptide bonds between amino acids, a water molecule is always lost in the process. Reactions, which involve the loss of water molecules during the process, are referred to as condensation reactions, and it implies that the formation of peptides through the coming together of the peptide bonds, is also a condensation reaction.
Single peptide bonds can be found between each amino acid pairing. Proteins are also known as polypeptides since they are made up of tens or hundreds of amino acids, which have been joined together with the peptide bonds.
Peptides bonds are broken through a process known as hydrolysis, which happens to be the exact opposite of condensation. The process involves the splitting of proteins into peptide chains, and a water molecule is always added to the process. But it should be noted that peptide bonds are very stable, and that it is never an easy task to break them buy peptides usa .
 Living With Healthy Hunger Health Blog
Living With Healthy Hunger Health Blog

